Course Overview
biomdsci 810: Biotechnology Operations
Year 1, Spring Semester (4 credits)
To succeed, biotechnology companies must juggle a host of complex technological and managerial functions.
This course takes a close-up look at seven interdependent functional specialties key to developing products for human health: regulatory affairs, quality assurance, biomanufacturing, quality control, non-clinical development, clinical development and project management.
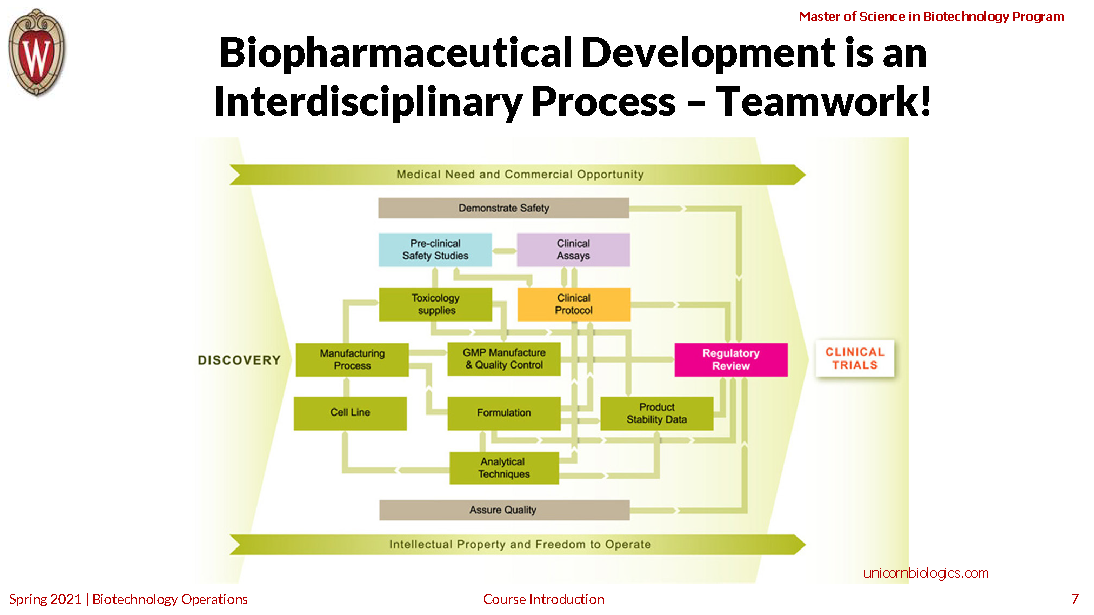
You’ll understand how companies plan, manage, coordinate and synchronize these disciplines to support a specific marketing plan—and how the underlying principals and practices are relevant to product development in other areas of biotechnology.
You’ll also learn how to design a development strategy, communicate objectives and lead a team through implementation.
Session Topics from Spring 2025
Session 1: Course Introduction; Overview of Product Development; Introduction to Quality; Introduction to Biomanufacturing; Quality Systems; Regulatory Submissions Overview
Session 2: Biomanufacturing: Process Design; Fermentation & Cell Culture; Concentration & Purification; Aseptic Processing & Sterilization; and CMC for Biological Formulations
Session 3: Quality Control: Specifications & Procedures; Biomanufacturing: Facilities, Equipment, Utilities; and Biomanufacturing: Qualification & Validation
Session 4: Group Lectures: Current Issues in Biotechnology; Clinical Development, Good Clinical Practice, and Clinical Operations
Session 5: Guest Speaker: Veterinary Medicines; Nonclinical Development: Pharmacology & Toxicology; Nonclinical Quality: Good Laboratory Practice; Nonclinical Development: Applied Principles; and Managing non-FDA Regulatory Compliance
Session 6: Regulatory Science: Roles & Responsibilities and Meetings & Submissions; Guest Speakers: Regulatory Issues & Perspectives in Biotechnology, Issues in Clinical Research
Session 7: Team Project Presentations


